| Uses |
An experimental drug meant to control lipids and increase the level of HDL, or good cholesterol, in the bloodstream.A cell-permeable, thiazolyl compound that acts as a potent, high affinity, PPARδ agonist. Exhibits selectivity for PPARδ compared to PPARα and PPARγ. Does not exibit any activity against other nuclear or non-nuclear receptors. Reported to increase cholesterol efflux and ABAC1 expression in macrophages, fibroblasts, and intestinal cells. GW 501516 has been found to enhance endurance in mice without any training. |
| Synthesis |
Add Cs2CO3 (3.25 g, 10.0 mmol), followed by the chloromethylthiazole (2.60 g) to a stirred solution of 4-mercapto-2-methylphenol (1.40 g) in CH3CN (80 mL). Stir the reaction mixture at room temperature for 4 hours. Add Cs2CO3 (4.89 g, 15.0 mmol), followed by methyl bromoacetate (1.23 mL, 13.0 mmol) to the mixture. Stir the reaction mixture at room temperature for another 5 hours. Pour the mixture into water. Extract the mixture with EtOAc (3 x 100 mL). Combine the organic layers. Wash the organic layers with brine. Dry the organic layers (Na2SO4). Concentrate the organic layers. Purify the residue by column chromatography on silica gel with hexane/ ethyl acetate (5:1). 1H NMR (CDCl3) δ7.97 (d, 2H, J= 8.4 Hz), 7.65 (d, 2H, J= 8.4 Hz), 7.21 (d, 1H, J= 2.4 Hz), 7.13 (dd, 1H, J= 8.4, 2.4 Hz), 6.58 (d, 1H, J= 8.4 Hz), 4.63 (s, 2H), 4.11 (s, 2H), 3.78 (s, 3H), 2.24 (s, 3H), 2.20 (s, 3H). 13C NMR (CDCl3) δ169.2, 163.0, 156.3, 151.3, 136.8, 136.1, 132.1, 131.2 (q, J= 32 Hz), 130.6, 128.4, 126.3, 125.8 (q, J= 4 Hz), 125.3, 122.1, 111.4, 65.4, 52.2, 32.4, 16.1, 14.8. 19F NMR (CDCl3) δ115.5 (s).
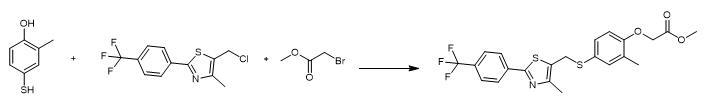
Fig. The synthetic step 1 of GW-501516 |



